The U.S. FDA recently approved a new drug, Taltz (ixekizumab), for the treatment of patients suffering from moderate-to-severe plaque psoriasis.
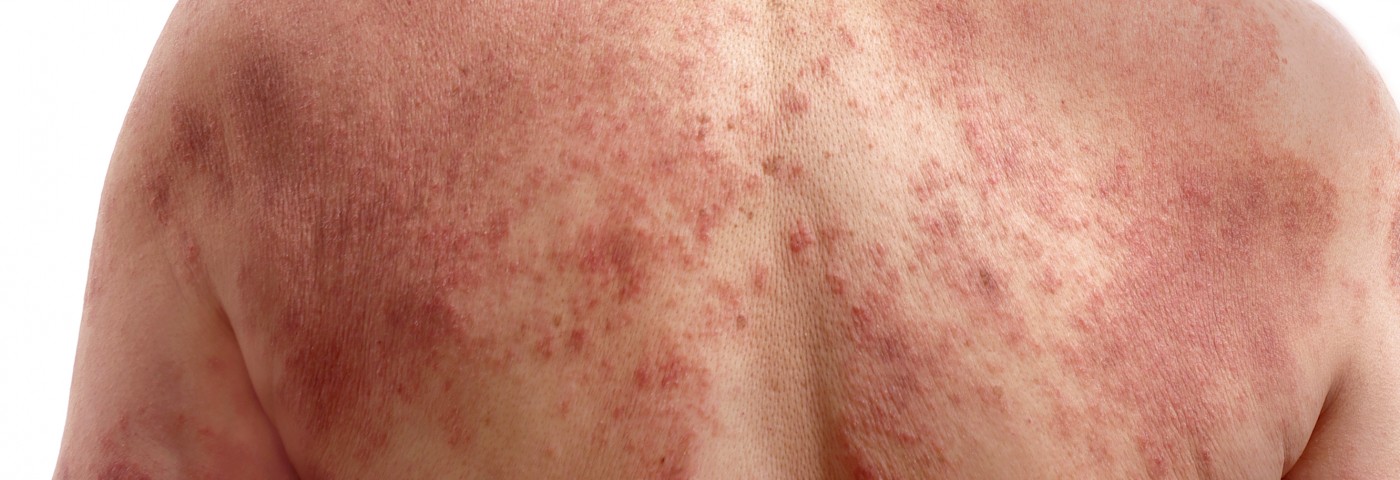
Psoriasis is a long-lasting autoimmune disease characterized by patches of abnormal skin such as red patches or flaking. It occurs more frequently in people who have a family history of the condition. The disease usually begins in people between the ages of 15-35.
The most frequent type of psoriasis, plaque psoriasis, or psoriasis vulgaris, makes up about 90 percent of cases. It typically presents with red patches with white scales on top. Areas of the body most commonly affected are the back of the forearms, shins, around the belly button, and the scalp.
“Today’s approval provides patients suffering from plaque psoriasis with another important treatment option to help relieve the skin irritation and discomfort from the condition,” stated Julie Beitz, director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research.
The active ingredient of Taltz is an antibody (ixekizumab) that can inhibit an inflammatory protein, interleukin (IL)-17A, which plays an important role in the development of the disease.
Administration of Taltz, marketed by Eli Lilly, is through injection for patients who are also candidates for systemic therapy (orally or injected treatments distributed through the bloodstream), phototherapy (ultraviolet light treatment), or both.
The safety and efficacy of ixekizumab was demonstrated in three randomized, placebo-controlled clinical studies of 3,866 participants suffering from plaque psoriasis. The participants were also candidates for systemic or phototherapy therapy. Results of the clinical trials confirmed that Taltz had better clinical response compared to placebo by measuring the changes in the extent, nature, and severity of the symptoms.
Because Taltz is a drug that influences the immune responses, it was approved with a Medication Guide that provides information about an increased risk of an infection or allergy, or autoimmune malfunctions. Side effects such as serious allergic reactions and development or worsening of inflammatory bowel disease were reported with Taltz. The most frequent side effects were upper pulmonary infections, injection site reactions, and fungal (tinea) infections.