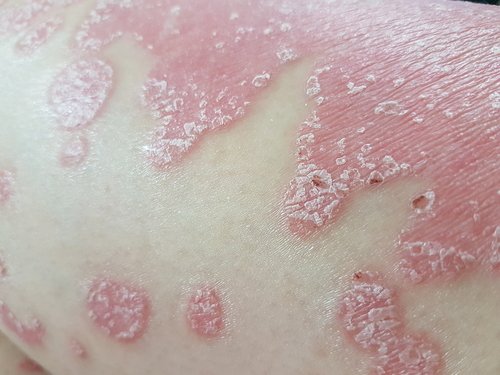
An already started Phase 2b clinical trial of Prurisol as a treatment for moderate-to-severe chronic plaque psoriasis has recruited more than 70 percent of the 189 participants that researchers hoped for.
The therapy’s developer, Cellceutix Corporation, said it expects to release the trial’s initial results during the third quarter of 2017.
Prurisol is an oral immunomodulatory therapy that reduces the expression of an RNA molecule called PRINS whose function has yet to be determined. PRINS stands for Psoriasis susceptibility-related RNA Gene Induced by Stress. Expression is the process by which information from a gene is used to create a functional product like a protein.
Cellceutix is following a regulatory path that it hopes can accelerate Prurisol’s U.S. approval. The Federal Drug Administration allows a company to use long-term safety results of an approved drug that is similar to its own when it seeks its candidate’s approval. In this case, the drug is a Prurisol bioequivalent called Ziagen.
Researchers have completed a 12-week Phase 2 clinical trial (NCT02494479) of Prurisol in patients with mild-to-moderate chronic plaque psoriasis. Two Prurisol doses were tested, with the study showing that the higher one improved patients’ psoriasis. The therapy was also well tolerated, the trial showed.
The Phase 2b trial that Cellceutix is now conducting (NCT02949388) is randomized, double-blind, parallel-group and placebo-controlled. It is assessing the drug’s effectiveness at doses higher than the 200 mg per day that researchers found to be successful in the previous trial.
Researchers are testing doses of 300 and 400 mg per day in the new trial. Patients who are already enrolled are already receiving one of the two.
The team is using patients’ Psoriasis Area and Severity Index (PASI) scores at the start and finish of the 12-week testing period to evaluate Prurisol’s effectiveness.
The trial’s primary goal is a 75% reduction in PASI scores over the 12 weeks. An interim analysis of effectiveness will be done after six weeks.
The effectiveness of current psoriasis treatments diminishes over time, and the therapies have side effects.
Cellceutix expects Prurisol to prove safe and effective not only for chronic plaque psoriasis but also for psoriatic arthritis, rheumatoid arthritis and other conditions.

I’ve used about everything on the market and love dermalmd psoriasis serum. When it came out recently I bought it at Walgreens and then couldn’t get it again as it was out of stock. So, Amazon to the rescue again and at a cheaper price! Psoriasis is very complicated, what works for one person may very well not work for another. I’ve spent much more on other products that did absolutely nothing, this worked for me, thank goodness.
Prurisol sounds amazing for a oral pill taken every 12 hours.
“…Among patients with the severest form of psoriasis in study, those having a baseline IGA score of 3 (“moderate”), the primary endpoint was met in 46% of patients who received 200 mg per day. These data were derived from analyses of all patients….”
Subjects in the current trial are getting either 400 mg/day, 300 mg/day or a placebo.
See pages 10 through 29 at the following link
https://static1.squarespace.com/static/5715352e20c647639137f992/t/5865318920099e2bc425d577/1483026833173/Cellceutix_Corporate_Slide+Deck_DERMATOLOGY_November+2016+Website+Vers.pdf
The Prurisol Phase 2b trial is currently ongoing and still recruiting patients at 29 sites across the USA.
https://clinicaltrials.gov/ct2/show/NCT02949388?term=NCT02949388&rank=1
Good luck and GOD bless,
George
They need to figure out a way to send the researchers to the patient instead of the patient to the researchers.
I have both psoriasis and psoriatic arthritis but live in the sticks.
Donna,
I hope that you find a good treatment.
Do you live near any of the 29 trial sites that are currently recruiting for the Prurisol trial?
Locations
United States, Arizona
Study Site Recruiting
Glendale, Arizona, United States, 85308
Clinical Study Site Recruiting
Rogers, Arizona, United States, 72758
United States, Arkansas
Study Center Recruiting
Hot Springs, Arkansas, United States, 71913
United States, California
Clinical Study Site Recruiting
Murrieta, California, United States, 92562
Clinical Study Site Recruiting
Oceanside, California, United States, 92056
Study Site Recruiting
Sherman Oaks, California, United States, 91403
United States, Colorado
Study Site Recruiting
Denver, Colorado, United States, 80209
Clinical Study Site Recruiting
Denver, Colorado, United States, 80210
United States, Florida
Study Site Recruiting
Port Orange, Florida, United States, 32127
Clinical Study Site Recruiting
Tampa, Florida, United States, 33609
United States, Illinois
Study Center Recruiting
Arlington Heights, Illinois, United States, 60005
United States, Indiana
Clinical Study Site Recruiting
South Bend, Indiana, United States, 46617
United States, Kansas
Study Center Recruiting
Overland Park, Kansas, United States, 66215
United States, Kentucky
Study Site Recruiting
Louisville, Kentucky, United States, 40217
United States, Michigan
Clinical Study Site Recruiting
Clarkston, Michigan, United States, 48346
Clinical Study Site Recruiting
Clinton, Michigan, United States, 48038
United States, Missouri
Clinical Study Site Recruiting
Saint Louis, Missouri, United States, 63117
United States, New Hampshire
Clinical Study Site Recruiting
Portsmouth, New Hampshire, United States, 03801
United States, New Jersey
Clinical Study Site Recruiting
Berlin, New Jersey, United States, 08009
United States, New York
Clinical Study Site Recruiting
New York, New York, United States, 10012
United States, Oregon
Clinical Study Site Recruiting
Portland, Oregon, United States, 97210
United States, Rhode Island
Study Site Recruiting
Johnston, Rhode Island, United States, 02919
United States, Texas
Study Center Recruiting
Austin, Texas, United States, 78759
Clinical Study Site Recruiting
Houston, Texas, United States, 77004
Clinical Study Site Recruiting
Pflugerville, Texas, United States, 78660
Clinical Study Site Recruiting
San Antonio, Texas, United States, 78213
Clinical Study Site Recruiting
San Antonio, Texas, United States, 78229
Study Site Recruiting
San Antonio, Texas, United States, 78229
United States, Virginia
Study Center Recruiting
Charlottesville, Virginia, United States, 22911
https://clinicaltrials.gov/ct2/show/NCT02949388?term=NCT02949388&rank=1
Good luck and GOD bless,
George
I have severe psoriasis on the bottom of both feet. I saw this and decided to try dermalmd serum. Slowly, by applying at least twice a day, my feet are healing and don’t hurt from being so raw.
My dermatologist suggested the Dermalmd serum so for me that worked just fine. I sometimes use breastmilk, so both together are the best combo
For me nothing worked as well as the dermalmd psoriasis cream, glides on the skin prevents irritation and i even dont itch anymore