Pfizer’s rheumatoid arthritis drug Xeljanz (tofacitinib) was effective against nail psoriasis in patients with plaque disease, according to data from two Phase 3 clinical trials.
The data further expands the known effects of the treatment, which also has been shown effective in other types of psoriasis.
The study, “Efficacy of tofacitinib for the treatment of nail psoriasis: Two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis,” was performed by Pfizer scientists in collaboration with academic researchers at the Brigham and Women’s Hospital of Harvard Medical School, among others. The work was published in the Journal of the American Academy of Dermatology.
The clinical trials (NCT01276639 and NCT01309737) had focused on plaque psoriasis, with earlier publications showing that Xeljanz was effective in this patient group.
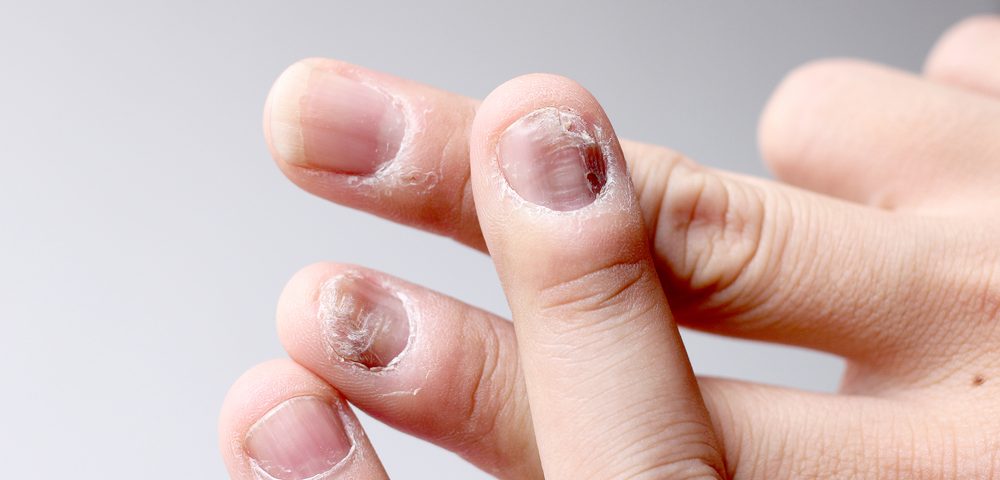
While nails make up only a small part of the body, researchers argued that psoriasis in the nails can have a relatively large impact on a patient’s quality of life.
So, they extracted data from the trials, looking at this specific aspect. The trials included nearly 1,200 patients who had nail psoriasis and were randomized to receive Xeljanz at two different doses, or placebo. The 5 mg and 10 mg Xeljanz dose-groups each held twice as many patients as the placebo group.
After 16 weeks, those receiving placebo were transferred to one of the two treatment groups. By then, more patients in the Xeljanz groups than in the placebo group had lowered their nail symptom severity scores by 50% or more.
Patients receiving the higher dose, lowered the score by 44.2%, those in the lower dose-group by 32.8%, and placebo-treated patients saw a 12% reduction.
Looking at the proportion of patients who lowered their severity scores by at least 75%, or by 100%, showed a similar pattern — the 10 mg Xeljanz dose was most effective, while placebo was least effective.
Measurements at week 28 showed that the treatment benefits continued to rise, and were mostly maintained when researchers checked back after 52 weeks of treatment.
Researchers, however, concede that the results might be biased because the trial protocols dictated that patients who did not experience a significant improvement in their psoriasis severity scores by week 28, should be excluded from further analyses.
Xeljanz has been approved in the U.S. for rheumatoid arthritis since 2012. After initially turning down the drug in 2013 due to safety concerns, the European Medicines Agency (EMA) approved the drug for the rheumatic condition in March this year.
Meanwhile, Pfizer has continued to study the drug in other conditions. After the successful completion of clinical trials in psoriatic arthritis, the company recently submitted a supplemental New Drug Application to the U.S. Food and Drug Administration (FDA) to expand its previously approved use to include psoriatic arthritis.
Several of the researchers reported financial ties to pharmaceutical companies other than Pfizer.


Nothing gets rid of psoriasis but foderma serum actually helped relieve itching, redness and scaliness better than many other products I’ve used. Recommend.